Background: One of the standards of intensive induction therapy for young patients with acute myeloid leukemia (AML) consists of 7 days of cytarabine and 3 days of either idarubicin 12mg/m 2 (IDA) or daunorubicin 60-90mg/m 2 (DNR) (7+3). Midostaurin (MIDO) is added to 7+3 for patients with FLT3-mutated AML. The pivotal trial that led to the approval of MIDO (RATIFY) used 60mg/m 2 of DNR as the anthracycline of choice. Although a direct comparison of IDA with DNR 60mg/m 2 is lacking, randomized studies have shown comparable results with DNR 90mg/m 2 and IDA. It is unclear if the addition of MIDO to IDA-based induction leads to improved outcomes when compared to 7+3 (IDA) alone.
Methods: We conducted a retrospective analysis to compare outcomes of young patients (≤65y) with FLT3-mutated AML treated with IDA-based induction, with and without MIDO, at the University of Alabama at Birmingham (UAB) between January 2010 and Dec 2022.
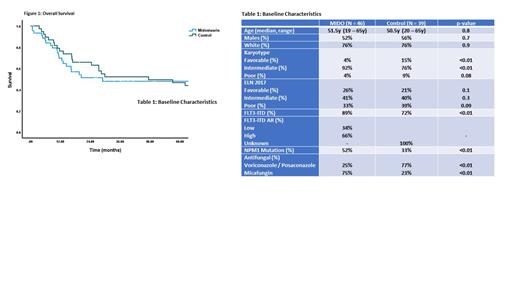
Results: There were 85 patients included in the analysis (MIDO=46, control=39). The baseline characteristics are shown in Table 1. The median age of the MIDO and control groups was 51.5y (19-65y) and 50.5y (20-65y), respectively. In the MIDO group, more patients had FLT3-ITD (89% vs. 72%, p<0.01) and NPM1 mutation (52% vs. 33%, p<0.01) compared to the control group. There was an overrepresentation of intermediate karyotype in the MIDO group (92% vs. 76%, p<0.01) and favorable karyotype in the control group (4% vs. 15%, p<0.01). Majority of the patients received micafungin (75%) in the MIDO group and either voriconazole or posaconazole (77%) in the control group.
All patients in the MIDO group received at least 1 dose and 70% of the patients completed all 14 days during induction. Ten patients had to discontinue MIDO early due to adverse events (gastrointestinal=6, hepatic=3, rash=1).
The composite complete remission (cCR) rate was higher in the control group (79%) compared to the MIDO group (71%) (p=0.01). In comparison, the protocol specified CR rate (by day 60) in the RATIFY trial was 59%. There were no significant differences between the two groups in terms of time to neutrophil and platelet recovery, breakthrough invasive fungal infections, febrile neutropenia and length of hospital stay.
In the MIDO group, 22 patients received MIDO during consolidation chemotherapy. Twenty-two out of 46 and 25/39 patients proceeded to allogeneic stem cell transplantation (allo-SCT) in the MIDO and control group, respectively. The 4-year overall survival (OS) for the MIDO group was 50%, compared to 51% for the control group (p=0.1) (Figure 1). The 4-year OS for patients <60y was 54% and 55% in the MIDO and control group, respectively.
Conclusion: This is the first study to compare the impact of adding MIDO to IDA-based induction versus IDA-based induction alone. Our findings suggest that IDA-based induction may result in a higher cCR rate, with or without the addition of MIDO, when compared to DNR 60mg/m 2 (as per RATIFY). The addition of MIDO to IDA-based induction did not result in increased toxicity. These results suggest that IDA-based induction, instead of DNR 60mg/m 2, should be considered for younger patients with AML. The added benefit of MIDO, in the context of 7+3 with IDA or even DNR 90mg/m 2, needs further investigation.
Disclosures
Worth:Blueprint, CTI BioPharma: Consultancy; University of Alabama at Birmingham: Current Employment. Bachiashvili:University of Alabama at Birmingham: Current Employment. Vachhani:Incyte, CTI BioPharma Corp, Blueprint Medicines: Speakers Bureau; Abbvie, Amgen, Blueprint Medicines, Cogent Biosciences, Incyte, CTI BioPharma Corp, Daiichi Sankyo, GlaxoSmith Kline, Karyopharm, Novartis, Pfizer, Genentech, Inc., Servier, Stemline, MorphoSys, LAVA therapeutics: Honoraria. Jamy:Ascentage: Other: Advisory Board Participation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal